19-10-2016

Documental Management integrated with the LIMS Opensource Documental Workflow : the integrity of the documentation of the management system can be guaranteed by the correlation between the points of the UNI CEI EN ISO / IEC 17025: 2005 and the related documents of the system, based on specific workflows that allow to track down the Leggi tutto l’articolo…
Leggi tutto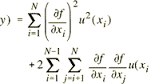
The Formule setting and logic to automate the calculation of the tests results or of Uncertainty value, is useful and powerful in speed and secure and traceable calculations. Sometimes, the formulas may be also useful for the determination of the list price starting from costs, or to select and combine the values of variables that Leggi tutto l’articolo…
Leggi tutto01-03-2016

Define the guidelines for the drafting of S.O.P. transposing parts of the regulations that require the Validation of Information Systems in support of critical activities (in particular those subject to GLP, cGMP, 21 CFR Part 11 FDA, UNI CEI EN ISO/IEC 17025/2005).
Leggi tutto